- Study protocol
- Open access
- Published:
Clinical effects of atorvastatin combined with conbercept in the treatment of patients with macular edema secondary to retinal vein occlusion and carotid plaque: study protocol for a prospective randomized controlled trial
Trials volume 25, Article number: 244 (2024)
Abstract
Introduction
Intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) drugs have been widely used in patients with macular edema (ME) secondary to retinal vein occlusion (RVO); however, recurrence is a major concern. This study aims to observe the clinical effects of atorvastatin and intravitreal therapy in the treatment of patients with branch or central RVO-ME and coexistent carotid plaques (CP).
Methods and analysis
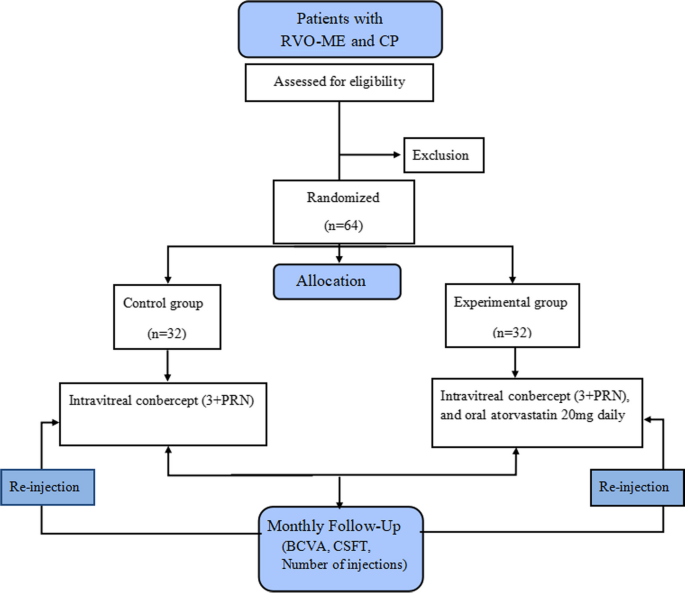
A prospective randomized controlled clinical trial will be conducted. Sixty-four patients diagnosed with branch or central RVO-ME and coexistent CP will be enrolled and randomly allocated in a 1:1 ratio to the control and experimental groups. The control group will be treated with intravitreal conbercept monthly for 3 months, followed by monthly evaluation and injection of pro re nata (PRN) for 12 months, while the experimental group will be treated with oral atorvastatin 20 mg daily combined with the control group treatment. If a drop of best-corrected visual acuity (BCVA) is more than five Early Treatment Diabetic Retinopathy Study (ETDRS) letters (one line) or an increment in central subfield thickness (CSFT) of 100 μm (or a 10% increment from the previous visit), intravitreal re-treatment will be performed. Outcome measurements include CSFT, BCVA, number of injections, and incidence of adverse events during the 12-month follow-up period. Differences between groups will be evaluated using Student’s t-test, and comparisons between groups will be evaluated using repeated-measures analysis of variance.
Ethics and dissemination
The study has been approved by the Institutional Review Board of Nanjing Lishui People’s Hospital, Nanjing, China (approval number 2023KY0418-12, dated 18 April 2023), and has been registered on chictr.org.cn. Written informed consent will be collected from each patient and the results of this trial will be submitted to a peer-reviewed journal.
Trial registration
Chinese Clinical Trial Registry ChiCTR2300071359. Registered on 12 May 2023.
Introduction
Retinal vein occlusion (RVO) is the second most common retinal vascular disease and is characterized by a sudden painless visual reduction [1, 2]. However, the exact mechanisms underlying RVO remain unclear. Recent evidence has demonstrated that RVO is closely associated with carotid plaques (CP), an important risk factor for stroke [3]. Evidence has confirmed that CP is observed in 54.3% and 76.7% of patients with RVO and branch RVO, respectively [3, 4]. Retinal arteriosclerosis and thrombosis caused by CP can result in retinal venous reflux disorders and secondary RVO [3,4,5]. RVO can be a predictor of CP and should be evaluated using carotid Doppler ultrasound [1]. Carotid Doppler ultrasound is non-invasive, useful for screening and diagnosing CP, and can accurately evaluate carotid intima-media thickness [3]. However, it is not commonly used for the clinical treatment of RVO.
Macular edema (ME) is the most severe and frequent complication of RVO [2]. Intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) drugs (e.g., ranibizumab, aflibercept, and conbercept) have been widely used as first-line treatment for patients with RVO-ME [2, 6,7,8]. However, they have several limitations. First, the clinical effect of anti-VEGF drugs is not long-lasting, and the recurrence of ME indicates repeated injections. Hunt et al. reported that the median number of injections of ranibizumab and aflibercept for branch RVO-ME was seven over 12 months [2]. Sun et al. used conbercept to treat RVO-ME and the mean number of injections was 7.14 ± 1.90 in branch RVO and 7.59 ± 1.39 in central RVO, over 9 months [8]. Second, frequent injections increase surgical risks, including endophthalmitis and secondary glaucoma [6]. Third, anti-VEGF drugs are expensive, and reinjections lead to an economic burden for patients. Therefore, recurrence during follow-up is a major concern for ophthalmologists [7]. Risk factors such as retinal structures (e.g., elevated central retinal thickness and disorganization of retinal inner layers) have been described; however, to the best of our knowledge, investigations of systemic factors such as CP in RVO-ME have not been discussed in the “EURETINA Guidelines for the Diagnosis and Treatment of Retinal Vein Occlusion” [9].
Clinical trials have shown that regular statin use plays a significant role in preventing cerebrovascular atherosclerosis and reducing the risk of cerebrovascular accidents [10]. Atorvastatin, a statin (lipid-lowering drug), has proven to be effective in stabilizing and reversing plaques in patients with carotid atherosclerosis in combination with antiplatelet agents [11,12,13]. Furthermore, a multicentre study showed that atorvastatin significantly reduced the risk of cardiovascular and cerebrovascular accidents in patients with CP, and the risks of stroke and coronary artery accidents decreased by 33% and 43%, respectively [14].
Recently, atorvastatin has been proven useful in reducing retinal VEGF expression and inhibiting inflammation [15, 16]. Oral atorvastatin was effective in patients with diabetic ME and age-related macular degeneration [17, 18]. Similarly, intravitreal statins can help reduce the development of proliferative vitreoretinopathy [19]. However, clinical investigations of atorvastatin in the treatment of RVO-ME and CP in the current studies are lacking.
Methods and analysis
Study objectives
This study aims to observe the clinical effects of atorvastatin and intravitreal therapy in the treatment of patients with branch or central RVO-ME and coexistent CP.
Trial design
In this trial, 64 patients diagnosed with branch or central RVO-ME and coexistent CP will be enrolled and randomly allocated in a 1:1 ratio to the control and experimental groups. The control group will be treated with intravitreal conbercept (Chengdu Kanghong Pharmaceutical; National Drug Approval No. S20130012), using the 3+ pro re nata (PRN) scheme (monthly for 3 months, followed by monthly evaluation and injection PRN for 12 months) and the experimental group will be treated with oral atorvastatin (Beijing Jialin Pharmaceutical Co., Ltd., GYZZH20093819) 20 mg daily combined with the control group treatment.
Eligibility criteria
Patients diagnosed with RVO-ME and CP are based on indirect ophthalmoscopy, fundus fluorescein angiography (FFA), spectral-domain optical coherence tomography (SD-OCT), and carotid Doppler ultrasound.
Inclusion criteria
The inclusion criteria are as follows: (1) patients with a branch or central RVO, aged between 45 and 75 years old; (2) central sub-field thickness (CSFT) > 250 μm, best corrected visual acuity (BCVA) < 0.5; and (3) body mass index < 28 kg/m2, carotid stenosis without recanalization surgery.
Exclusion criteria
The exclusion criteria are as follows: (1) patients with severe cataract, macular membrane, optic neuropathy, glaucoma, and uveitis; (2) patients with a branch or central RVO-ME in both eyes; (3) patients with macular edema secondary to age-related macular degeneration, diabetic retinopathy, Coats disease, Eales disease, or high myopia; (4) history of intravitreal injection, retinal surgery, laser photocoagulation, or ocular trauma; (5) history of taking lipid-lowering drugs in the past month; (6) patients with severe cardiovascular, cerebrovascular, liver, and kidney dysfunction; and (7) patients who cannot tolerate surgery or have poor compliance.
Withdrawal criteria
The withdrawal criteria are as follows: (1) the follow-up was lost; (2) informed consent was withdrawn; and (3) decision to withdraw for severe adverse events.
Methodology
A prospective, randomized controlled clinical trial will be conducted at Nanjing Lishui People’s Hospital. Patients diagnosed with branch or central RVO-ME and CP will be enrolled in this study. They will be randomly allocated in a 1:1 ratio to the control and experimental groups. The study design is illustrated in Fig. 1.
Randomization
Patients will be randomly divided into two groups in a 1:1 ratio using SPSS (Statistical Package for Social Sciences, version 26.0, IBM, Armonk, NY, USA). An independent investigator will generate a random sequence and allocate interventions.
Masking
Patients and clinicians will be blinded after assignment. The implementation and maintenance of the randomization and masking methods will be completely validated. To avoid evaluation bias, the treatment groups will be hidden from the ophthalmologist who collects the clinical data during each follow-up period. Analysts dealing with the data will also be masked.
Interventions
All patients will undergo preoperative and postoperative investigations. Systemic examinations include routine blood tests, blood biochemistry, infection measurements (HIV, hepatitis B and C, and syphilis), chest computed tomography (CT), electrocardiography, and carotid Doppler ultrasound. Ophthalmic examinations include BCVA, slit-lamp, intraocular pressure, indirect ophthalmoscopy, FFA, and SD-OCT. The timeline of data collection is shown in Table 1.
Outcome measurements
Monthly follow-ups will be performed after surgery in both groups. The necessary investigations will be conducted at each follow-up visit.
The outcome measurements include CSFT, BCVA, number of injections, and incidence of adverse events.
Primary outcomes
The primary outcome is the average change in CSFT from baseline to 12 months.
Secondary outcomes
Secondary outcomes are BCVA, average number of injections, and incidence of adverse events at each visit during the 12-month follow-up visit.
Safety and combined operation-related adverse events
Possible operation-related complications include elevated intraocular pressure, endophthalmitis, traumatic cataract, vitreous hemorrhage, retinal hemorrhage, retinal detachment, and drug-related adverse events (e.g., liver function damage and gastrointestinal discomfort). In this trial, all the above adverse events and possible causes will be recorded and managed accordingly.
Follow-up plan
Monthly follow-ups and necessary re-examinations after surgery will be conducted. BCVA, slit lamp, intraocular pressure, indirect ophthalmoscopy, and SD-OCT will be performed at each visit. Blood biochemistry and carotid Doppler ultrasonography will be performed every 3 months. Routine blood tests, FFA, and electrocardiograms will be performed every 6 months. Infection measurements (HIV, hepatitis B and C, and syphilis) and chest CT will be performed during the last visit. The number of injections and the incidence of complications will be recorded and evaluated at each follow-up.
Re-injection criteria
If the BCVA drops by more than five Early Treatment Diabetic Retinopathy Study (ETDRS) letters (one line) or an increment in CSFT of 100 μm (or a 10% increment from the previous visit), intravitreal re-treatment will be performed.
Sample size calculation
The sample size is estimated as follows: according to the results from our pre-experiment, the average CSFT in the control and experimental groups was 263.72 ± 30.63 μm and 241.17 ± 18.54 μm, respectively. The type I error rate (β) was set at 0.05, with 80% power at 5% significance and 1:1 randomization. Zα = 1.96, Zβ = 0.84, standard deviation (σ) in experimental group = 30.63, difference in means (δ) = 263.72 − 241.17 = 22.55.
Sample size \(n=\frac{2{\left({z}_a+{z}_{\beta}\right)}^{2\ast }{\sigma}^2}{\delta^2}=2\times {\left(1.96+0.84\right)}^2\times 30.{63}^2/22.{55}^2=29.\)
Thus, 29 patients will be required in each group. Considering a loss ratio of 10%, the total sample size will increase to 32 patients in each group.
Statistical analysis
Data from the patients’ clinical records will be processed using SPSS. For normally distributed data, continuous variables will be expressed as mean ± standard deviation, difference between groups will be evaluated by the Student’s t-test, and comparisons inter-group at each endpoint will be evaluated by repeated measures analysis of variance. The Bonferroni method will be used to adjust the overall significance level for multiplicity. For abnormally distributed data, continuous variables will be expressed as M (Q1, Q3), differences between groups will be evaluated using the Mann–Whitney U test, and comparisons between groups at each endpoint will be evaluated using a generalized linear mixed model. Categorical variables will be expressed as counts (%), and the chi-square test will be performed. The effect of missing data on the trial results will be assessed using sensitivity analyses of the augmented datasets. Dropouts will be included in the analysis by using modern imputation methods for missing data. The BCVA will be converted to the minimum resolution angle in logarithmic form before data analysis. The clinical data of the branch and central RVO will be sub-analyzed. Two-tailed tests of significance will be performed, and P-values < 0.05 will be considered statistically significant.
Provisions for post-trial care
There is no anticipated harm and compensation for trial participation.
Ethics and dissemination
The study has been approved by the institutional review board of Nanjing Lishui People’s Hospital, Nanjing, China (approval number 2023KY0418-12, dated 18 April 2023) and registered with the Chinese clinical trial registry (http://www.chictr.org.cn/, No. ChiCTR2300071359). Written informed consent will be collected from each patient. They will be informed about the study procedures, possible treatment risks, and their right to withdraw from the trial. The results of this trial will be submitted to a peer-reviewed journal.
Access to the full protocol, participant-level dataset and statistical code
The datasets analyzed in the current study and the statistical code are available from the corresponding author upon reasonable request, as is the full protocol.
Patient public involvement
The patients and the public were not involved in the design, conduct, reporting, or dissemination of our research plans.
Discussion
This is a prospective, randomized controlled clinical trial investigating the clinical effect of atorvastatin and intravitreal conbercept in the treatment of ME in patients with a branch or central RVO-ME and coexistent CP. We speculate that the treatments in the experimental group may have significantly improved the carotid intima-media thickness and inhibited VEGF and inflammation in the retina. This study may provide an opportunity to reduce the recurrence rate of RVO-ME. Further studies with larger sample sizes and more data are required to better understand the effects of combined treatments.
Oversight and monitoring
Composition of the coordinating center and trial steering committee
The trial steering committee will be formed by the principal investigator and the co-investigators. The committee will manage the entire project and submit a report for publication at the end of the study. The committee will appoint inspectors of the Project Management Group once a month. The project management group will oversee standardization in line with clinical practice requirements and submit their reports to the trial steering committee.
Composition of the data monitoring committee, its role and reporting structure
A member will be designated by the principal investigator to handle the data. Electronic paper case report forms and management tools will be used to collect the data. The trial steering committee managed the trial under the supervision of the project management group.
Frequency and plans for auditing trial conduct
The project management group will meet every 2 weeks to review the trial. The Trial Steering Group and Ethics Committee will monitor throughout the trial period. Owing to the low-risk nature of the intervention, an independent Data Monitoring Committee will not be considered.
Protocol amendments
If there are any changes to the protocol, the sponsor and funder will be notified first, the principal investigator will notify the centers, and a copy of the revised protocol will be sent to the principal investigator to be added to the investigator-site file. Any deviations from the protocol will be fully documented using a breach report. This protocol will be updated in the clinical trial registry.
Trial status
At the time of manuscript submission, this trial has recruited 14 patients. This trial will be completed by December 2024. The current protocol (Code: ChiCTR2300071359) is version 1.0, dated 18 April 2023.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BCVA:
-
Best corrected visual acuity
- IOP:
-
Intraocular pressure
- SD-OCT:
-
Spectral-domain optical coherence tomography
- FFA:
-
Fluorescence fundus angiography
- CT:
-
Computed tomography
- RVO:
-
Retinal vein occlusion
- ME:
-
Macular edema
- VEGF:
-
Vascular endothelial growth factor
- CP:
-
Carotid plaque
- CSFT:
-
Central subfield thickness
- ETDRS:
-
Early Treatment Diabetic Retinopathy Study
References
Bozkurt E, Cetin T, Rencuzogullari I. Evaluation of Systemic Endothelial Dysfunction in Retinal Vein Occlusions. Beyoglu Eye J. 2022;7:126–33.
Hunt AR, Nguyen V, Creuzot-Garcher CP, Alforja S, Gabrielle PH, Zarranz-Ventura J, et al. Twelve-month outcomes of ranibizumab versus aflibercept for macular oedema in branch retinal vein occlusion: data from the FRB! registry. Br J Ophthalmol. 2022;106:1178–84.
Yu L, Duan Z, Tao T, Xu S, Mo N, Jia Y, et al. The correlation between carotid stenosis detected by neck vascular ultrasound and branch retinal vein occlusion. Zhonghua Yan Ke Za Zhi. 2014;50:804–7 Chinese.
Lyu M, Lee Y, Kim BS, Kim HJ, Hong R, Shin YU, et al. Clinical significance of subclinical atherosclerosis in retinal vein occlusion. Sci Rep. 2021;11:11905.
Sanlés González I, Napal Lecumberri JJ, Pérez-Montes R, Cerveró Varona A, Casado Rojo A, Hernández Hernández JL. Retinal vein occlusion in patients under 50 years. Analysis of vascular risk factors, thrombophilia, carotid ultrasound findings and uncommon aetiologies. Arch Soc Esp Oftalmol (Engl Ed). 2022;97:443–9.
Corazza P, D’Alterio FM, Savastano MC, Kabbani J, Duguid G, Savastano A, et al. Long-term outcomes of anti-VEGF treatment of macular oedema due to retinal vein occlusions. Eur J Ophthalmol. 2022;32:3536–46.
Xing Q, Dai YN, Huang XB, Peng L. Comparison of efficacy of conbercept, aflibercept, and ranibizumab ophthalmic injection in the treatment of macular edema caused by retinal vein occlusion: a Meta-analysis. Int J Ophthalmol. 2023;16:1145–54.
Sun Z, Zhou H, Lin B, Jiao X, Luo Y, Zhang F, et al. Efficacy and safety of intravitreal conbercept injections in macular edema secondary to retinal vein occlusion. Retina. 2017;37:1723–30.
Schmidt-Erfurth U, Garcia-Arumi J, Gerendas BS, Midena E, Sivaprasad S, Tadayoni R, et al. Guidelines for the Management of Retinal Vein Occlusion by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2019;242:123–62.
Yoon M, Lee CJ, Park S, Lee SH. Statins and Clinical Outcomes in Patients With Low to Moderate Risk but With Non-obstructive Carotid Plaques: The SCOPE-CP Study. Korean Circ J. 2022;52:890–900.
Deng Y, Zou W, Chen G, Shangguan S, Zhou F, Jiang W, et al. Comparative studies on the effects of different doses of atorvastatin combined with aspirin on inflammatory cytokines and carotid plaques in patients with ischemic cerebrovascular disease. Int J Neurosci. 2019;129:1133–8.
Liping Z, Xiufang L, Tao Y, Baomin Z, Houshuai T. Efficacy comparison of rosuvastatin and atorvastatin in the treatment of atherosclerosis and drug safety analysis. Pak J Pharm Sci. 2018;31:2203–8.
Zhu YC, Jiang XZ, Bai QK, et al. Evaluating the Efficacy of Atorvastatin on Patients with Carotid Plaque by an Innovative Ultrasonography. J Stroke Cerebrovasc Dis. 2019;28:830–7.
Sillesen H, Amarenco P, Hennerici MG, Callahan A, Goldstein LB, Zivin J, et al. Stroke Prevention by Aggressive Reduction in Cholesterol Levels Investigators. Atorvastatin reduces the risk of cardiovascular events in patients with carotid atherosclerosis: a secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2008;39:3297–302.
Tsujinaka H, Itaya-Hironaka A, Yamauchi A, Sakuramoto-Tsuchida S, Shobatake R, Makino M, et al. Statins decrease vascular epithelial growth factor expression via down-regulation of receptor for advanced glycation end-products. Heliyon. 2017;3:e00401.
Tian B, Al-Moujahed A, Bouzika P, Hu Y, Notomi S, Tsoka P, et al. Atorvastatin promotes phagocytosis and attenuates pro-inflammatory response in human retinal pigment epithelial cells. Sci Rep. 2017;7:2329.
Gupta A, Gupta V, Thapar S, Bhansali A. Lipid-lowering drug atorvastatin as an adjunct in the management of diabetic macular edema. Am J Ophthalmol. 2004;137:675–82.
Ozkiris A, Erkiliç K, Koç A, Mistik S. Effect of atorvastatin on ocular blood flow velocities in patients with diabetic retinopathy. Br J Ophthalmol. 2007;91:69–73.
Mysore Y, Del Amo EM, Loukovaara S, Hagström M, Urtti A, Kauppinen A. Statins for the prevention of proliferative vitreoretinopathy: cellular responses in cultured cells and clinical statin concentrations in the vitreous. Sci Rep. 2021;11:980.
Acknowledgements
Not applicable.
Strengths and limitations of this study
➢ This is a prospective, randomized controlled clinical trial investigating the clinical effects of atorvastatin and intravitreal conbercept in the treatment of patients with RVO-ME and coexistent CP.
➢ This trial may provide an opportunity to control ME recurrence in patients with RVO and concomitant CP.
➢ Data on carotid intima-media thickness in this trial were not collected or analyzed.
➢ Further studies with larger sample sizes and more data are required to better understand the effects of combined treatments.
Role of sponsor
The sponsor played no part in the study design, collection, management, analysis, interpretation of data, writing of the report, or decision to submit the report for publication.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
BTY wrote the manuscript; BW edited the manuscript; JY contributed to the supervision; YG was responsible for the analysis; HY contributed to the study design, YHL and GL consulted literature and dealt with the table; BTY and XYW established the diagnosis and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of Nanjing Lishui People’s Hospital (Approval number 2023KY0418-12, dated 18 April 2023). All participants will sign informed consent before the study, and all methods will be performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yao, B., Wang, B., Yang, J. et al. Clinical effects of atorvastatin combined with conbercept in the treatment of patients with macular edema secondary to retinal vein occlusion and carotid plaque: study protocol for a prospective randomized controlled trial. Trials 25, 244 (2024). https://doi.org/10.1186/s13063-024-08082-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-024-08082-0