- Study protocol
- Open access
- Published:
An optimised dosing regimen versus a standard dosing regimen of vancomycin for the treatment of late onset sepsis due to Gram-positive microorganisms in neonates and infants aged less than 90 days (NeoVanc): study protocol for a randomised controlled trial
Trials volume 21, Article number: 329 (2020)
Abstract
Background
Vancomycin has been used in clinical practice for over 50 years; however, validated, pharmacokinetic (PK) data relating clinical outcomes to different dosing regimens in neonates are lacking. Coagulase negative staphylococci (CoNS) are the most commonly isolated organisms in neonatal, late-onset sepsis (LOS). Optimised use to maximise efficacy while minimising toxicity and resistance selection is imperative to ensure vancomycin’s continued efficacy.
Methods
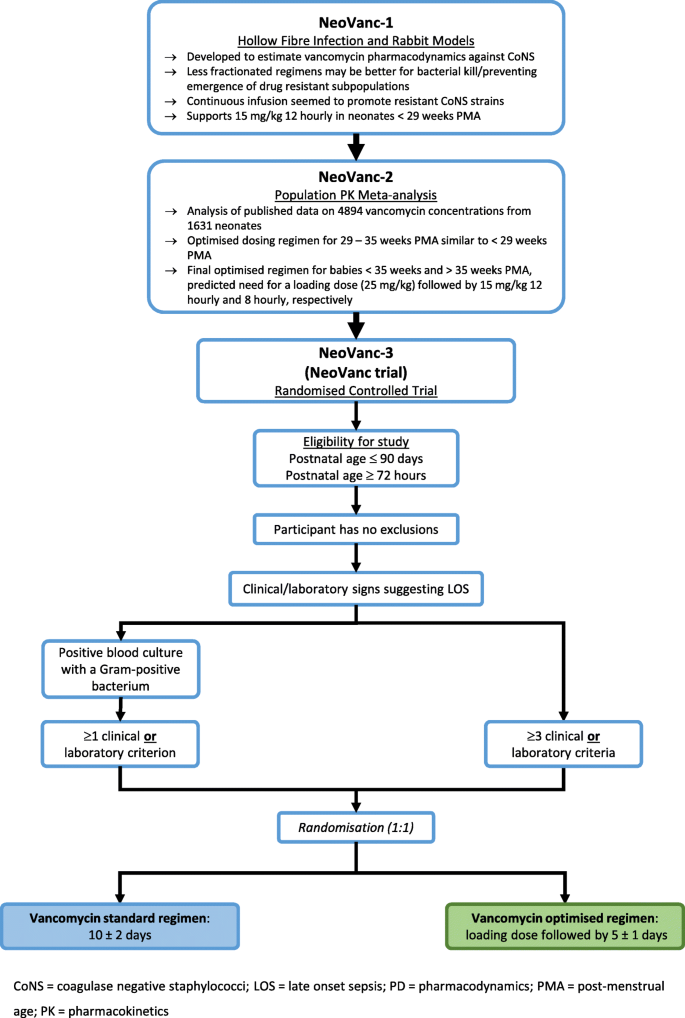
NeoVanc is a European, open-label, Phase IIb, randomised, controlled, non-inferiority trial comparing an optimised vancomycin regimen to a standard vancomycin regimen when treating LOS known/suspected to be caused by Gram-positive organisms (excluding Staphylococcus aureus) in infants aged ≤ 90 days. Three hundred infants will be recruited and randomised in a 1:1 ratio. Infants can be recruited if they have culture confirmed (a positive culture from a normally sterile site and at least one clinical/laboratory criterion) or clinical sepsis (presence of any ≥ 3 clinical/laboratory criteria) in the 24 h before randomisation.
The optimised regimen consists of a vancomycin loading dose (25 mg/kg) followed by 5 ± 1 days of 15 mg/kg q12h or q8h, dependent on postmenstrual age (PMA). The standard regimen is a 10 ± 2 day vancomycin course at 15 mg/kg q24h, q12h or q8h, dependent on PMA.
The primary endpoint is a successful outcome at the test of cure visit (10 ± 1 days after the end of vancomycin therapy). A successful outcome consists of the patient being alive, having successfully completed study vancomycin therapy and having not had a clinical/microbiological relapse/new infection requiring treatment with vancomycin or other anti-staphylococcal antibiotic for > 24 h.
Secondary endpoints include clinical/microbiological relapse/new infection at the short-term follow-up visit (30 ± 5 days after the initiation of vancomycin), evaluation of safety (renal/hearing), vancomycin PK and assessment of a host biomarker panel over the course of vancomycin therapy.
Discussion
Based on previous pre-clinical data and a large meta-analysis of neonatal, PK/pharmacodynamic data, NeoVanc was set up to provide evidence on whether a loading dose followed by a short vancomycin course is non-inferior, regarding efficacy, when compared to a standard, longer course. If non-inferiority is demonstrated, this would support adoption of the optimised regimen as a way of safely reducing vancomycin exposure when treating neonatal, Gram-positive LOS.
Trial registration
ClinicalTrials.gov, NCT02790996. Registered on 7 April 2016.
EudraCT, 2015–000203-89. Entered on 18 July 2016.
Background
Neonatal late-onset sepsis
Neonatal late-onset sepsis (LOS) is defined as sepsis occurring 48–72 h after birth. The causative organisms associated with neonatal LOS tend to be those found in the neonatal intensive care unit (NICU) or are normal skin or gut commensals. Coagulase negative staphylococci (CoNS) are the most commonly recovered organisms from the blood cultures of neonates with LOS. Overall mortality rates secondary to CoNS are reported as < 2% in resource-rich settings; however, this increases to ~ 9% in very low birth weight (VLBW) and preterm infants [1,2,3,4]. CoNS-associated LOS has a significant morbidity with episodes of LOS having an adverse effect on neurodevelopmental outcomes as well as increasing the cost of hospitalisation, primarily due to prolonged length of stay [1, 5,6,7,8]. Interventions on the NICU are often invasive, e.g. central venous catheters, and these can increase a neonate’s exposure to hospital-acquired pathogens such as CoNS [1].
Diagnosis of neonatal sepsis
Diagnosing neonatal sepsis can be difficult, particularly as clinical signs of sepsis can frequently overlap with disorders unrelated to sepsis [9]. Blood culture positivity rates in neonates are low [10] and pharmacodynamic (PD) markers, such as C-reactive protein (CRP) can demonstrate variable results in neonates, particularly preterm and VLBW babies [11, 12]. Novel methods for diagnosing neonatal sepsis such as biomarkers based on host RNA are under development. A prospective, case-control study provided clinically validated evidence that host RNA from whole blood can serve as a causally relevant biomarker for detecting neonatal infection [13, 14].
Vancomycin use and emerging antimicrobial resistance
Glycopeptides are the most commonly prescribed antibiotics in children for hospital-acquired infections in Latin America, North America, Australia and Europe, with vancomycin being one of the most widely utilised antibiotics in the treatment of Gram-positive sepsis. Methicillin and gentamicin resistances in CoNS are reported as 85% and in the range of 32%-94%, respectively [15,16,17,18]; vancomycin is, therefore, a first-line therapy in sepsis due to CoNS [19].
It is well-known that high rates of antibiotic use are associated with antimicrobial resistance [20,21,22]. The wide variation in vancomycin dosing and monitoring practices seen across NICUs demonstrates that optimal dosing and monitoring regimens are not well standardised in this population [23] and highly variable between countries and centres [24]. One UK study revealed 17 different combinations of dose, timing of dose and timing of monitoring [25]. Current vancomycin-dosing regimens recommended for preterm neonates, such as those in the European Society for Paediatric Infectious Diseases Manual of Childhood Infection – The Blue Book and the British National Formulary for Children, are based on expert opinion or on studies including only a small number of babies rather than on data generated from clinical trials [26,27,28].
The wider impact of different vancomycin-dosing regimens and course durations on normal, neonatal, gut and skin flora are not known. However, antibiotic use can lead to the disruption of the microbiome leading to colonisation with resistant bacteria and candida [5, 29, 30]. Colonisation with vancomycin-resistant enterococci and candida are known risk factors for invasive infection with associated morbidity and mortality [31, 32].
Trial concept
In view of the lack of validated, neonatal, vancomycin pharmacokinetic (PK), safety and efficacy data to support optimal use, the European Medicines Agency (EMA) included vancomycin on its ‘Revised priority list for studies into off-patent paediatric medicinal products’. The EMA called for more data on the optimal dosing and monitoring regimens for preterm and term neonates with sepsis caused by staphylococci and non-pyogenic streptococci [33]. It was advised that new data should be generated from PK/PD and safety-based efficacy studies. The NeoVanc project has been developed in direct response as a planned drug discovery programme. The discovery and licencing of new antibiotics is a lengthy process; therefore, ascertaining optimal dosing of the older, off-patent vancomycin is essential to ensure the continued efficacy of this important antibiotic in the treatment of neonatal LOS [34].
Methods and trial design
Objectives
The primary objective of the NeoVanc trial is to compare the efficacy of an optimised vancomycin-dosing regimen to a standard vancomycin-dosing regimen in patients with LOS, known or suspected to be caused by Gram-positive microorganisms.
The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist is provided in Additional file 1.
Study design and selection of optimised dosing regimen
The NeoVanc clinical trial (www.neovanc.org) is an open-label, European, multi-centre, Phase IIb, randomised, active control, parallel group, non-inferiority trial. A total of 300 participants are expected to be enrolled from approximately 20 NICUs in five European Union countries (Estonia, Greece, Italy, Spain and the United Kingdom).
The NeoVanc project forms part of a Paediatric Investigation Plan (PIP) which has received a favourable opinion from the EMA Paediatric Committee (PDCO). The clinical trial, as part of the PIP, aims to provide data on dosing, efficacy and safety of vancomycin in neonates, which will lead to a Paediatric-Use Marketing Authorisation (PUMA) application.
The pre-clinical studies—(1) a hollow-fibre infection model, a rabbit model [35] and a staphylococcal biofilm in vitro model [36]; and (2) a population pharmacokinetic meta-analysis of neonatal, vancomycin studies [37] to define the optimal dosing regimen for the clinical trial—are detailed in Fig. 1.
Ethical approval has been gained in all participating European countries as well as approvals from the relevant Regulatory and Ethics Authorities.
Study participants
Infants admitted to the NICU with suspected/confirmed LOS. Participants can be recruited if they fulfil the inclusion criteria and have none of the exclusion criteria.
Inclusion criteria
The inclusion criteria for the clinical trial are adapted from the European Medicines Agency definition for neonatal LOS [38].
Postnatal age ≤ 90 days at randomisation
AND
Postnatal age ≥ 72 h at onset of sepsis
AND
Clinical sepsis as defined by presence of any three clinical or laboratory criteria from the list below, in the 24 h before randomisation
OR
Confirmed, significant bacterial sepsis as defined by positive culture with a Gram-positive bacterium from a normally sterile site and at least one clinical or one laboratory criterion from the list below, in the 24 h before randomisation.
Clinical criteria
-
Hyperthermia or hypothermia
-
Hypotension or impaired peripheral perfusion or mottled skin
-
Apnoea or increased oxygen requirement or increased requirement for ventilatory support
-
Bradycardic episodes or tachycardia
-
Worsening feeding intolerance or abdominal distension
-
Lethargy or hypotonia or irritability
Laboratory criteria
-
White blood cell (WBC) count < 4 or > 20 × 109 cells/L
-
Immature to total neutrophil ratio (I/T) > 0.2
-
Platelet count < 100 × 109/L
-
CRP > 10 mg/L
-
Glucose intolerance as defined by a blood glucose value > 180 mg/dL (> 10 mmol/L) when receiving normal glucose amounts (8–15 g/kg/day)
-
Metabolic acidosis as defined by a base excess (BE) < − 10 mmol/L (< − 10 mEq/L) or a blood lactate value > 2 mmol/L
Exclusion criteria
-
Administration of any systemic antibiotic regimen for > 24 h before randomisation, unless the change is driven by the apparent lack of efficacy of the original regimen
-
Treatment with vancomycin for ≥ 24 h at any time within 7 days of randomisation
-
Known toxicity, hypersensitivity or intolerance to vancomycin
-
Known acute renal impairment as defined by urinary output < 0.7 mL/kg/h for 24 h or a creatinine value ≥ 10 μmol/L (1.13 mg/dL)
-
Patient receiving (or planned to receive) haemofiltration, haemodialysis, peritoneal dialysis, extracorporeal membrane oxygenation (ECMO) or cardiopulmonary bypass
-
Severe congenital malformations where the infant is not expected to survive for > 3 months
-
Patient known to have S. aureus (MSSA or MRSA) bacteraemia
-
Patient with osteomyelitis, septic arthritis, urinary tract infection (UTI) or meningitis
-
Patient with high suspicion of/confirmed sepsis caused by Gram-negative organisms or fungi
-
Other situations where the treating physician considers a different empiric antibiotic regimen necessary
-
Current participation in any other clinical study of an investigational medicinal product (IMP)
Post-randomisation exclusions
-
Any participant found to have Gram-negative or fungal sepsis, osteomyelitis, septic arthritis, urinary tract infection, meningitis or S. aureus (MSSA or MRSA) bacteraemia after randomisation will be excluded from the efficacy analysis but followed up for safety.
Dosing schedule by arm
Standard regimen: 15 mg/kg q24h (PMA < 29 weeks), q12h (PMA 29–35 weeks) or q8h PMA > 35 weeks. Duration is 10 ± 2 days. Optimised regimen: all participants receive a single loading dose of 25 mg/kg followed by a maintenance dose of 15 mg/kg q12h (PMA ≤ 35 weeks) or q8h (PMA > 35 weeks). Duration is 5 ± 1 days. Dose adjustments should only occur within the context of routine therapeutic drug monitoring. The only exception is if renal impairment develops, when study vancomycin dose may be adjusted dependent on vancomycin levels/local practice.
Concomitant antibiotics
Use of concomitant antibiotics during vancomycin therapy is allowed except for specific anti-staphylococcal agents (flucloxacillin, oxacillin, linezolid, tedizolid, daptomycin and teicoplanin).
Assignment of interventions
Infants will be randomised in a 1:1 ratio to either the standard treatment regimen or the optimised treatment regimen. Randomisation will be managed via a secure web-based randomisation facility. The randomisation program will use a minimisation algorithm to ensure balance between the groups with respect to clinical centre, PMA at randomisation, and presence or absence of an umbilical catheter/central venous line.
Local investigators and participants’ parents/guardians will not be blinded to regimen allocation as this is an open label study. Investigators undertaking the biomarker and microbiological sub-analyses will be blinded to arm allocation. The trial management group and trial data analysts will be blinded to aggregate outcomes from interim analyses; unblinding will, therefore, not occur.
Primary outcome measure
The primary outcome incorporates clinical and microbiological factors. Successful outcome at the Test of Cure (TOC) visit (10 ± 1 days after the end of actual vancomycin therapy [EVT])
Participant is alive
and
Successful outcome at EVT (defined as the participant is alive, there is a significant improvement in the participant’s overall, clinical status, there is microbiological resolution or presumed eradication of bacteria and no new vancomycin-susceptible pathogens potentially associated with sepsis have been identified)
and
Participant has not had a clinically or microbiologically significant relapse or new infection, requiring treatment with vancomycin or other specific anti-staphylococcal antibiotics (flucloxacillin, oxacillin, linezolid, tedizolid, daptomycin and teicoplanin) for > 24 h within 10 days of EVT visit
Secondary outcome measures
-
Evaluation of efficacy
-
Clinically or microbiologically, significant relapse or new infection within 10 days of EVT, requiring treatment with any antibiotic (other than vancomycin or specific anti-staphylococcal antibiotics [flucloxacillin, oxacillin, linezolid, tedizolid, daptomycin and teicoplanin]) for > 24 h
-
Clinically significant relapse or new infection at short-term follow-up (STFU; 30 ± 5 days from initiation of study vancomycin) visit
-
-
Evaluation of safety by allocation group
-
Abnormal renal function tests at the STFU visit
-
Abnormal hearing screening test
-
Comparative safety of vancomycin (related to all other parameters other than renal and hearing safety) at STFU visit
-
-
PK/PD evaluation
-
PK parameters of vancomycin using population PK modelling by allocation group
-
Probability of target attainment with different study regimens
-
-
Microbiological evaluation
-
Relationship between CoNS species and duration of treatment and CRP response
-
Gut, skin and mucosal colonisation by vancomycin resistant organisms at baseline, EVT and STFU visit
-
Bacterial DNA polymerase chain reaction (PCR) analysis in babies ≥ 29 weeks PMA
-
-
Biomarker evaluation
Study procedures
Recruitment and consent
The parent(s)/guardian(s) of potential participants are approached by research staff to assess eligibility, provide information and obtain informed consent. Informed consent is only gained after information has been provided, questions have been answered and time has been given for consideration. If consent is gained, the informed consent form is signed by the parents/guardians before study recruitment according to the national ethical regulations of the participating countries. Parents/guardians can also pre-consent to the trial. In this case, the same procedure applies; however, parents/guardians are approached before their baby becomes unwell. If pre-consent is in place and a baby develops signs of sepsis and meets the eligibility criteria, the parents/guardians can be contacted via telephone or in person to confirm continued consent.
Consent for the trial as well as the PK and microbiological sub-analyses are gained together; information is provided in relation to all sub-analyses in the participant information sheet and informed consent form. Participants’ parents/guardians will also be asked for permission for the research team to share relevant data with people from the organisations taking part in the research or from regulatory authorities, where relevant. A copy of the informed consent form can be provided by the corresponding author on request.
Visits
Eight visits are planned, from the neonate’s screening and randomisation to the audiology follow-up visit and will be performed as outlined in Table 1 – Schedule of Study Assessments for the NeoVanc Clinical Trial.
Monitoring of clinical and laboratory parameters
Participants will be monitored for specific clinical signs and laboratory parameters in line with the EMA’s criteria for the diagnosis of neonatal sepsis, at day 3, day 5 ± 1 and day 10 ± 2 (standard arm only) [38], so as to determine the rate of recovery. At EVT, an assessment will be made according to specified criteria as to whether the baby has had a significant improvement in their overall, clinical status. Participants who have met this criteria will proceed to the TOC visit where an assessment to determine whether the baby has had a clinically significant new infection (three clinical or laboratory signs), microbiological relapse (positive blood culture with phenotypically similar organism + one clinical/laboratory sign) or microbiological new infection (positive blood culture with an organism of interest + one clinical/laboratory sign) which has required > 24 h of anti-staphylococcal antibiotics since the EVT visit. All participants will undergo hearing screening.
Study-specific assessments
Blood isolates
Blood culture will be collected at baseline and subsequently if positive. Isolates will be stored and sent to the central microbiology laboratory for identification and antimicrobial susceptibility testing. Relevant isolates will be tested for biofilm production and biofilm susceptibility.
Bacterial DNA PCR
Bacterial DNA PCR will be utilised in the identification of causative organisms where the blood culture is negative, as well as quantitative analysis as a PD marker. Bacterial DNA analysis will only take place in those infants who are ≥ 29 weeks PMA in view of the restrictions on the blood volumes allowed in clinical trials. Samples are taken at baseline, Visit 3 and the STFU visit.
Disruption of normal colonising flora
Stool (or rectal swab if stool unavailable) as well as axilla and nasal swabs will be collected at baseline, EVT and at the STFU visit to assess the impact of vancomycin on gut, skin and mucosal flora. Babies will be screened for vancomycin-resistant enterococci, candida and a subset for CoNS with reduced susceptibility to vancomycin, which will all be used as surrogate markers of disruption to normal flora. It will be determined whether colonisation with these organisms changes over the treatment course, as well as between specific groups, e.g. preterm versus term babies.
Biomarkers
A set of biomarkers will be performed as a classifier with high accuracy in predicting specifically bacterial infection. Biomarkers samples are taken at baseline, visits 3, 4, 5 (standard arm only) and the STFU visit.
Sample size
This is a non-inferiority study comparing a standard regimen and an optimised regimen. It is hypothesised that 95% of the participants in the standard treatment arm will not require a second course of treatment with anti-staphylococcal antibiotics and that the proportion will be the same in the optimised treatment group. Using the Wilson-score method, a sample size of 150 babies per arm will give at least 90% of power to demonstrate a non-inferiority margin of 10% (nQuery Software, ‘Statsols’; Statistical Solutions Ltd., Cork, Ireland).
Statistical analysis
Analysis of the primary outcome will be performed using binomial regression with an identity link to report the risk difference and its 95% confidence interval (CI) (risk of a successful outcome at TOC visit in the optimised regimen – risk in the standard regimen). The optimised regimen will be considered non-inferior if the lower limit of the 95% CI is ≥ − 10%. Non-inferiority of the optimised regimen will be tested using a one-sided test at the 2.5% level of significance.
An unadjusted analysis will be carried out as well as an adjusted analysis, adjusting for the minimisation factors used during randomisation by including them as covariates – centre, PMA at randomisation (< 29 weeks/29–35 weeks/> 35 weeks), and presence or absence of an umbilical catheter/central venous line. If there are insufficient participants in some centres to use this as a covariate factor, the centre will be used to adjust the variance estimator by including it as a clustering factor. This analysis will be carried out using both per-protocol (PP) and intention-to-treat (ITT) populations for comparison but the primary inference will be based on the adjusted analysis of the PP population.
For the secondary outcomes, risk ratios and their 95% CIs will be estimated using a log binomial regression model or using a log Poisson regression model with a robust variance estimator if the binomial model fails to converge. The analyses will be carried out for both the PP and ITT populations and, in each case, an unadjusted and adjusted analysis will be carried out as described for the primary outcome.
Pre-specified subgroup analysis will be performed for the primary outcome using the statistical test of interaction, based on PMA at randomisation, birth weight category, and presence or absence of an umbilical catheter/central venous line at the onset of sepsis.
Pharmacokinetics analysis
Pharmacokinetic data will be analysed by nonlinear mixed-effect modelling method (NONMEM). The vancomycin plasma concentration data will be imported into R for exploratory analysis and formatted for subsequent modelling using NONMEM version 6.2 (Globomax, USA). An interim PK analysis will be performed when PK and scavenged samples have been obtained from 50 participants.
Adverse event reporting
All adverse events occurring from the administration of the first dose of study vancomycin to the final follow-up visit will be recorded. In addition, serious adverse events, which meet the criteria for expedited reporting, will be reported to the Sponsor within 24 h of the awareness of the event.
Data management
Data will be collected in an electronic case report form which will be managed by Consorzio per Valutazioni Biologiche e Farmacologiche. All data collected will remain strictly confidential and anonymous. Access to the data is mandated by the Sponsor.
End of trial
The end of trial will be the point of database lockdown.
Committees
Network Management Group (NMG) is an executive body comprising of NeoVanc Work Package leaders and the Trial Statistician with decision powers over the whole NeoVanc project, its technical development and work plan updates. The NeoVanc Trial Management Group is a subgroup of the NMG and comprises of the individuals actively involved in this clinical trial. The group is responsible for managing the operational aspects of the trial. The Independent Data Monitoring Committee (IDMC) will monitor progress, safety and PK data with the aim of protecting the safety and interests of the trial participants and scientific integrity of the trial. The IDMC is composed of a neonatologist, a microbiologist and a statistician. The members of the IDMC are independent and have no involvement with the NeoVanc clinical trial.
Monitoring
Monitoring of the study will be performed by Therakind Ltd. in accordance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice guidelines, local regulations and Therakind Standard Operating Procedures.
Audit can be performed at any time by independent, properly authorised individuals mandated by the Sponsor. The audit is aimed to ensure the quality of the research, data accuracy, and respect of laws and regulations.
Specific considerations
Study-specific procedures have been kept to as few as possible and the volumes of blood to be obtained fall well within the guidelines set out by the EMA [39]. Bacterial DNA analysis will only be undertaken in neonates ≥ 29 weeks PMA. Study procedures will be carried out at the same time as routine investigations, where possible, to minimise discomfort.
Neonatal sepsis is serious and requires rapid treatment initiation; therefore, it will not always be possible to gain consent for the study within this timeframe. Up to 24 h of antibiotic administration prior to randomisation has been allowed within the protocol to allow for this. However, pre-consent is also allowed where consent can be gained before a baby becoming unwell, allowing for IMP to be initiated as soon as possible.
Trial sponsor
The trial sponsor is Fondazione PENTA Onlus (Email: info@penta-id.org). The sponsor has not participated in the study design and will not participate in the collection, analysis or interpretation of the data.
Discussion
Clinical trials and neonates
The NeoVanc trial opted to adapt the EMA criteria for the diagnosis for neonatal sepsis, by allowing entry to the trial on three clinical or laboratory signs as opposed to requiring two clinical and two laboratory signs. The reason for this was due to the less severe presentation and milder sepsis course seen with CoNS [1,2,3,4].
The NeoVanc trial is an open-label study. The use of a placebo in neonatal trials is controversial particularly if preterm infants are included in recruitment. In studies evaluating intravenous medication, the use of placebo can be a challenge in view of the careful fluid balance required in this population. Blinding would be difficult in view of the differing course lengths unless a placebo was used for the remaining days in the short course arm. It is recognised by the NeoVanc Consortium that this could lead to ascertainment bias particularly in view of the varying prescribing practices for vancomycin throughout Europe; some clinicians may favour a short course over a long course or vice versa [23, 25]. Investigators will be strongly encouraged to adhere to the allocated vancomycin duration and an assessment of the baby’s clinical status will be undertaken on the day vancomycin is actually stopped as well as on the day vancomycin should have been terminated so as to ascertain if the baby fulfilled the protocol’s definition of recovered/significantly improved.
The NeoVanc trial provides the unique opportunity to evaluate multiple aspects of neonatal, vancomycin pharmacokinetics within one study. Different course lengths will be explored but also the use of a loading dose, in the optimised arm, will help to ascertain if vancomycin levels can be safely increased more quickly and thus allow course duration to be reduced. More frequent dosing in babies < 29 weeks PMA will also be assessed in the optimised arm where babies receive dosing every 12 h instead of every 24 h as in the standard arm.
Finding an acceptable balance between antibiotic resistance, safety, duration and efficacy is a major challenge when treating neonatal sepsis; the NeoVanc trial aims to focus on all four components.
Trial status
The trial opened to recruitment on 3 March 2017; the first participant was recruited on 17 March 2017. The current protocol version is 6.0 (dated 7 September 2016). Recruitment is expected to complete by 31 December 2019.
Dissemination policy
There are no current plans for granting public access to participant-level datasets or statistical code. Outcomes of this trial will be published in a peer-reviewed journal.
Abbreviations
- CI:
-
Confidence interval
- CoNS:
-
Coagulase negative staphylococci
- CRP:
-
C-reactive protein
- CVBF:
-
Consorzio per Valutazioni Biologiche e Farmacologiche
- ECMO:
-
Extracorporeal membrane oxygenation
- EMA:
-
European Medicines Agency
- EOAT:
-
End of allocated vancomycin therapy
- EVT:
-
End of actual vancomycin therapy
- IDMC:
-
Independent Data Monitoring Committee
- IMP:
-
Investigational Medicinal Product
- ITT:
-
Intention to treat
- LOS:
-
Late onset sepsis
- NICU:
-
Neonatal intensive care unit
- NIHR:
-
National Institute for Health Research
- NMG:
-
Network Management Group
- PCR:
-
Polymerase chain reaction
- PD:
-
Pharmacodynamics
- PDCO:
-
EMA Paediatric Committee
- PIP:
-
Paediatric Investigation Plan
- PK:
-
Pharmacokinetics
- PMA:
-
Postmenstrual age
- PP:
-
Per protocol
- STFU:
-
Short-term follow-up
- TOC:
-
Test of cure
- UTI:
-
Urinary tract infection
- WBC:
-
White blood cells
References
Isaacs D. A ten year, multicentre study of coagulase negative staphylococcal infections in Australasian neonatal units. Arch Dis Child Fetal Neonatal Ed. 2003;88(2):F89–93.
Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B. Epidemiological, clinical, and microbiological characteristics of late-onset sepsis among very low birth weight infants in Israel: a national survey. Pediatrics. 2002;109(1):34–9.
Cantey JB, Wozniak PS, Pruszynski JE, Sánchez PJ. Reducing unnecessary antibiotic use in the neonatal intensive care unit (SCOUT): A prospective interrupted time-series study. Lancet Infect Dis. 2016;16:1178–84.
Benjamin DK, DeLong E, Cotten CM, Garges HP, Steinbach WJ, Clark RH. Mortality following blood culture in premature infants: increased with Gram-negative bacteremia and candidemia, but not Gram-positive bacteremia. J Perinatol. 2004;24(3):175–80.
Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2):285–91.
Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff A. a, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–65.
Wheater M, Rennie JM. Perinatal infection is an important risk factor for cerebral palsy in very-low-birthweight infants. Dev Med Child Neurol. 2000;42(6):364–7.
Gray JE, Richardson DK, McCormick MC, Goldmann DA. Coagulase-negative staphylococcal bacteremia among very low birth weight infants: relation to admission illness severity, resource use, and outcome. Pediatrics. 1995;95(2):225–30.
Seale AC, Blencowe H, Manu AA, Nair H, Bahl R, Qazi SA, et al. Estimates of possible severe bacterial infection in neonates in sub-Saharan Africa, south Asia, and Latin America for 2012: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:731–41.
Kellogg JA, Ferrentino FL, Goodstein MH, Liss J, Shapiro SL, Bankert DA. Frequency of low level bacteremia in infants from birth to two months of age. Pediatr Infect Dis J. 1997;16(4):381–5.
Dritsakou K, Liosis G, Gioni M, Glynou E, Avdeliodi K, Papagaroufalis K. CRP levels in extremely low birth weight (ELBW) septic infants. J Matern Neonatal Med. 2015;28(2):237–9.
Lai M-Y, Tsai M-H, Lee C-W, Chiang M-C, Lien R, Fu R-H, et al. Characteristics of neonates with culture-proven bloodstream infection who have low levels of C-reactive protein (≦10 mg/L). BMC Infect Dis. 2015;15(1):320.
Smith CL, Dickinson P, Forster T, Craigon M, Ross A, Khondoker MR, et al. Identification of a human neonatal immune-metabolic network associated with bacterial infection. Nat Commun. 2014;5:1–15.
Dickinson P, Smith C, Forster T, Craigon M, Ross A, Khondoker M, et al. Whole blood gene expression profiling of neonates with confirmed bacterial sepsis. Genomics Data. 2015;3:41–8.
Hira V, Sluijter M, Estevao S, Horst-Kreft D, Ott A, de Groot R, et al. Clinical and molecular epidemiologic characteristics of coagulase-negative staphylococcal bloodstream infections in intensive care neonates. Pediatr Infect Dis J. 2007;26(7):607–12.
Brilene T, Soeorg H, Kiis M, Sepp E, Kõljalg S, Lõivukene K, et al. In vitro synergy of oxacillin and gentamicin against coagulase-negative staphylococci from blood cultures of neonates with late-onset sepsis. APMIS. 2013;121(9):859–64.
Klingenberg C, Sundsfjord A, Rønnestad A, Mikalsen J, Gaustad P, Flægstad T. Phenotypic and genotypic aminoglycoside resistance in blood culture isolates of coagulase-negative staphylococci from a single neonatal intensive care unit, 1989-2000. J Antimicrob Chemother. 2004;54(5):889–96.
Neumeister B, Kastner S, Bartmann P, Trautmann M, Marre R. Interpretive criteria for susceptibility testing of coagulase-negative staphylococci with special reference to netilmicin. Infection. 1997;25(3):175–7.
Versporten A, Bielicki J, Drapier N, Sharland M, Goossens H. ARPEC project group. The worldwide antibiotic resistance and prescribing in european children (ARPEC) point prevalence survey: Developing hospital-quality indicators of antibiotic prescribing for children. J Antimicrob Chemother. 2016;71(4):1106–17.
Lee VC. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm Ther. 2015;40(4):277–83.
Meyer E, Gastmeier P, Deja M, Schwab F. Antibiotic consumption and resistance: Data from Europe and Germany. Int J Med Microbiol. 2013;303:388–95.
Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 2014;14:13.
Metsvaht T, Nellis G, Varendi H, Nunn AJ, Graham S, Rieutord A, et al. High variability in the dosing of commonly used antibiotics revealed by a Europe-wide point prevalence study: implications for research and dissemination. BMC Pediatr. 2015;15:41.
Leroux S, Zhao W, Bétrémieux P, Pladys P, Saliba E, Jacqz-Aigrain E. Therapeutic guidelines for prescribing antibiotics in neonates should be evidence-based: A French national survey. Arch Dis Child. 2015;100(4):394–8.
Kadambari S, Heath PT, Sharland M, Lewis S, Nichols A, Turner MA. Variation in gentamicin and vancomycin dosage and monitoring in UK neonatal units. J Antimicrob Chemother. 2011;66(11):2647–50.
Sharland M. Blue Book Antimicrobial Dosing Guide. In: Manual of Childhood Infections - the Blue Book. 4th ed. Oxford: Oxford University Press; 2016. p. 965.
Paediatric Formulary Committee. British National Formulary for Children. London: BMJ Group; 2017.
de Hoog M, Mouton JW, van den Anker JN. Vancomycin: pharmacokinetics and administration regimens in neonates. Clin Pharmacokinet. 2004;43(7):417–40.
Rafii F, Sutherland JB, Cerniglia CE. Effects of treatment with antimicrobial agents on the human colonic microflora. Ther Clin Risk Manag. 2008;4:1343–58.
Antachopoulos C, Patel K, Sprague B, Short B, Campos J, Singh N. Risk factors for candidiasis in the neonatal unit: a matched case-control study. In: European Society of Clinical Microbiology and Infectious Diseases Conference; 2004. p. 362. (P1302). http://www.blackwellpublishing.com/eccmid14/clm_902_p3.pdf. Accessed 16 Aug 2018.
Yüce A, Karaman M, Gülay Z, Yulug N. Vancomycin-resistant Enterococci in neonates. Scand J Infect Dis. 2001;33(11):803–5.
Benjamin DK, Garges H, Steinbach WJ. Candida bloodstream infection in neonates. Semin Perinatol. 2003;27(5):375–83.
European Medicines Agency. Revised priority list for studies on off-patent paediatric medicinal products [Internet], vol. 44: Human Medicines Development and Evaluation; 2012. p. 1–12. https://www.ema.europa.eu/en.
Sertkaya A, Birkenbach A, Berlind A, Eyraud J. Examination of clinical trial costs and barriers for drug development. US Department of Health & Human Services - Office of the Assistant Secretary for Planning and Evaluation. 2014. https://aspe.hhs.gov/report/examination-clinical-trial-costs-and-barriers-drug-development. Accessed 18 Jun 2018.
Ramos-Martin V, Johnson A, Livermore J, McEntee L, Goodwin J, Whalley S, et al. Pharmacodynamics of vancomycin for CoNS infection: Experimental basis for optimal use of vancomycin in neonates. J Antimicrob Chemother. 2016;71(4):992–1002.
Simitsopoulou M, Kadiltzoglou P, Kyrpitzi D, Roilides E. Differential susceptibility of staphylococcus epidermidis biofilms to vancomycin, daptomycin and linezolid between clinical isolates from neonates and adults. Int J Biol Med Res. 2019;10(1):6591–6.
Jacqz-Aigrain E, Leroux S, Thomson A, Allegaert K, Capparelli E, Biran V, et al. Population pharmacokinetic meta-analysis of individual data to design the first randomized efficacy trial of vancomycin in neonates and young Infants. J Antimicrob Chemother. 2019;74(8):2128–38.
European Medicines Agency. Report on the Expert Meeting on Neonatal and Paediatric Sepsis [Internet], vol. 44; 2010. p. 1–6. https://www.ema.europa.eu/en/documents/report/report-expert-meeting-neonatal-paediatric-sepsis_en.pdf. Accessed 18 Jun 2018.
Committee for Medicinal Products for Human Use and Paediatric Committee. Guideline on the Investigation of Medicinal Products in the Term and Preterm Neonate [Internet]; 2010. p. 1–20. https://www.ema.europa.eu/en/investigation-medicinal-products-term-preterm-neonate. Accessed 18 Jun 2018.
Acknowledgments
We thank the parents/guardians of the participants of NeoVanc who have consented for their baby to be enrolled in the study.
Louise F Hill is supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South London at King’s College Hospital NHS Foundation Trust. The views expressed are those of the author[s] and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
On behalf of the NeoVanc Consortium: PENTA, Padova, Italy - Carlo Giaquinto, Davide Bilardi; St George’s, University of London, UK – Mike Sharland, Paul T Heath, Louise F Hill, Timothy Planche, Tatiana Munera Huertas; University of Liverpool, UK – Mark A Turner, William Hope; University of Tartu, Estonia – Irja Lutsar; Aristotle University, Thessaloniki, Greece – Emmanuel Roilides; Hôpital Robert Debré, Paris, France – Evelyne Jacqz-Aigrain, Wei Zhao; National Perinatal Epidemiology Unit, Oxford, UK – Louise Linsell; University of Edinburgh, UK – Peter Ghazal; Children’s Hospital Bambino Gesù, Rome, Italy – Andrea Dotta, Fundación Investigación Biomédica “Hospital 12 de Octubre”, Madrid Spain – Javier de la Cruz, Clara Alonso Díaz; Therakind Ltd., UK – Susan Conroy, Louise Rawcliffe; Consorzio per le Valutazioni Biologiche e Farmacologiche – Donato Bonifazi, Cristina Manfredi, Mariagrazia Felisi.
We would also like to thank Jean–Pierre Aboulker – INSERM Clinical Trials Unit, Paris, France.
Funding
This research has received funding from the European Union Seventh Framework Programme for research, technological development and demonstration under Grant Agreement No 602041. The funders have not participated in the study design and will not participate in the collection, analysis or interpretation of the data or preparation of future manuscripts.
Author information
Authors and Affiliations
Consortia
Contributions
LFH and MAT are the Trial Co-ordinators, drafted the manuscript and participated in the design of the study protocol. IL, PTH and ER drafted the manuscript and participated in the design of the study protocol. MS is the Chief Investigator for the study, participated in the design of the study and drafted the manuscript. EJ-A designed the pharmacokinetic aspects of the study and drafted relevant parts of the manuscript. PH participated in the design of the study and completed the statistical analyses section with LL. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The trial received a favourable opinion from the London - West London & GTAC Research Ethics Committee (REC reference: [16]/LO/1026) on 18 July 2016. Ethics Committee approvals have also been granted in Estonia, Greece, Italy and Spain, as well as Regulatory Body approvals in each country.
Any modifications/updates to trial documents will be communicated to trial personnel and study sites following the necessary ethical and regulatory approvals.
Written consent has been gained from all participant’s parents/guardians by trained research personnel; parents/guardians are free to withdraw their babies at any time.
Trials insurance is in place.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
SPIRIT 2013 Checklist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hill, L.F., Turner, M.A., Lutsar, I. et al. An optimised dosing regimen versus a standard dosing regimen of vancomycin for the treatment of late onset sepsis due to Gram-positive microorganisms in neonates and infants aged less than 90 days (NeoVanc): study protocol for a randomised controlled trial. Trials 21, 329 (2020). https://doi.org/10.1186/s13063-020-4184-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-020-4184-8