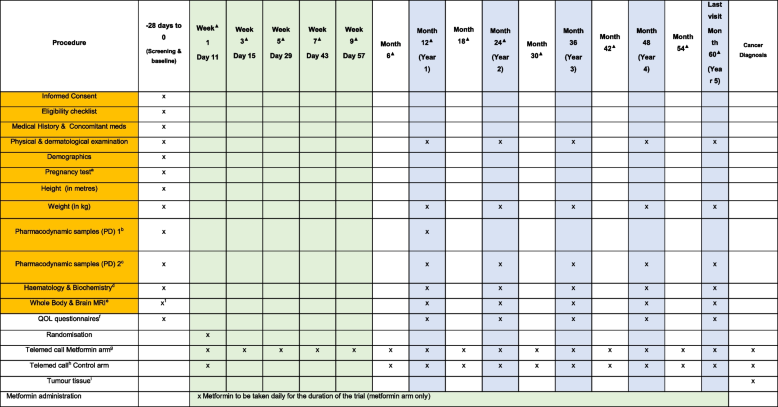
-
aPregnancy test (urine) for participants of child-bearing potential and who are sexually active
-
bPharmacodynamic sample PD1
-
cPharmacodynamic sample PD2
-
dFasting biochemistry and haematology blood: FBC (Hb, WBC, platelets), biochem (sodium, potassium, urea, creatinine, eGFR, liver function tests, vitamin B12 (baseline and year 5 only)) and for the translational research endpoints: insulin, glucose and IGF1 (baseline and M12)
-
eWhole body and brain MRI (WB-MRI) to be performed at the investigator (recruiting) sites. If a whole body and brain MRI scan has been completed in the 3 months prior to randomisation, this will be accepted as the baseline and screening scan.
-
fQuality of Life questionnaires: 12-item short form survey (SF12V2), Cancer Worry Scale & Treatment Burden Questionnaire (TBQ)
-
gTelemed call metformin arm*: Initial screening—explain randomisation outcome and call structure. Management of dose-titration phase, determine adverse events, changes to concomitant meds and review of adherence (MARS-5 Medication Adherence Questionnaire from month 6 onwards).
-
hTelemed call control arm*: Screening—explain randomisation outcome and call structure. From month 6 to determine adverse events, changes to concomitant meds.
-
iTumour tissue: Paraffin-embedded diagnostic tumour tissue or pre-cancerous tissue block
-
jMetformin administration: metformin will be sent by post from the recruiting hospital pharmacy
-
*For both arms, additional telemed calls may be made by the telemed team