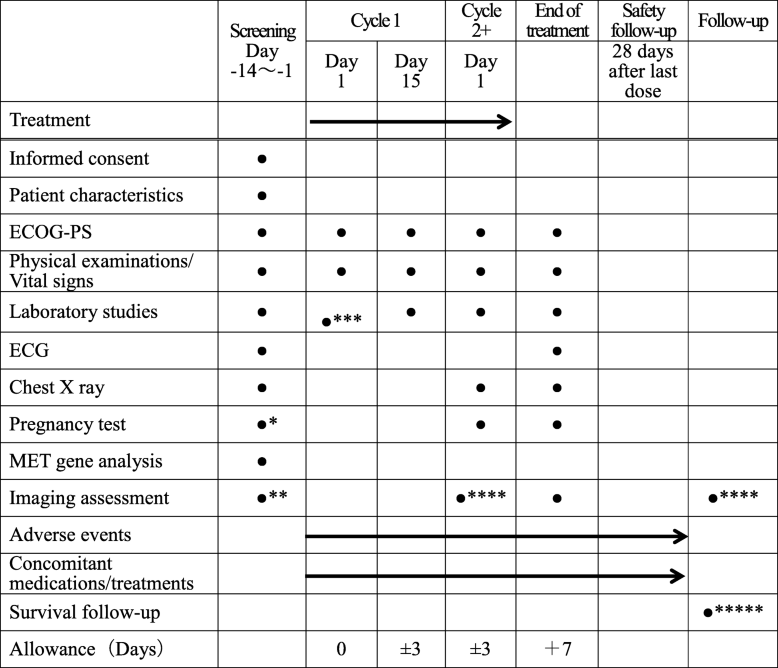
- * After Cycle 1 Day 1, pregnancy test should be performed when pregnancy is suspected
- ** Imaging assessments (Contrast CT of chest, abdomen, and pelvis and brain CT or MRI scan with contrast) will be performed at Day −28 to Day −1 prior to Cycle 1 Day 1. Bone MRI is not required unless clinically indicated
- *** Laboratory studies can be substituted with data carried out within 7 days
- **** Every 8 weeks and, after 12 cycles, every 12 weeks, assessment will be performed using CT and/or MRI scan (+/− 7 days window). Chest X-rays are not required at the time of CT examination. Patients who discontinue prior to RECIST v1.1-defined progressive disease will continue with tumor assessments according to the protocol until disease progression is documented or initiation of additional anticancer therapy
- ***** Survival information will be collected every 3 months until death, loss to follow-up, or withdrawal of consent for survival. The investigator will collect survival information until 1 year after the last patient has enrolled into the study