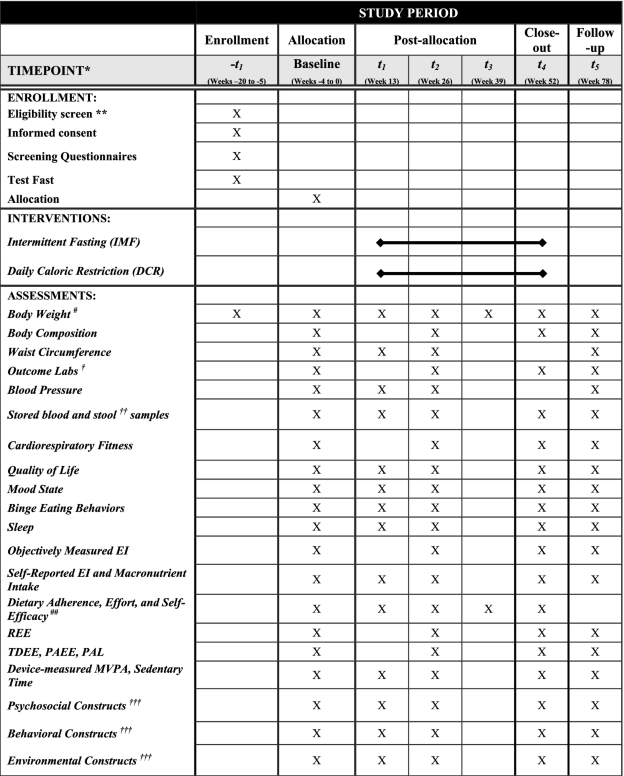
- EI energy intake, REE resting energy expenditure, TDEE total daily energy expenditure, PAEE physical activity energy expenditure, PAL physical activity level, MVPA moderate-to-vigorous physical activity
- *All measures, post-allocation, will occur ±2 weeks from when the measure is due
- **Screening measures include blood draw (complete blood count (CBC)), comprehensive metabolic panel (CMP), lipid profile, thyroid stimulating hormone (TSH), and hemoglobin A1C, electrocardiogram, height in centimeters (stadiometer), and physical exam and medical history
- #Outcome weights will be taken in the morning, after an overnight fast, with the participant wearing a hospital gown. In the IMF group, weight will be taken in the morning following a fed day. Weight will also be measured weekly during weeks 0–26 and every other week during weeks 27–52 at the group-based behavioral weight loss sessions
- ##These measures will be collected monthly
- †Outcome labs include glucose, insulin, triglycerides (TG), free fatty acids (FFA), beta-hydroxybutyrate (BHB), and cortisol. These will be measured at baseline after both a fed (i.e., 12-h fast) and fast day (i.e., 36-h fast, with exception of 25% EI). A lipid panel, insulin, glucose, hemoglobin A1C, leptin, ghrelin, peptide YY, highly sensitive C-reactive protein (hs-CRP), and brain-derived neurotrophic factor (BDNF) will be performed at baseline and weeks 26 and 52
- †† Stored blood and stool samples will be collected at baseline and weeks 13, 26, 52, and 78 in subjects who consented to sample storage
- †††See Table 4 for a detailed list of each construct for psychosocial, behavioral, and environmental factors

