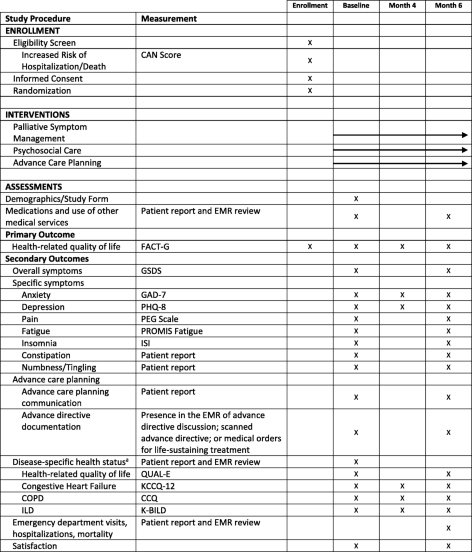
- Adapted from SPIRIT (Standard Protocol Items Recommendations for Interventional Trials) figure. SPIRIT checklist included in Additional file 1.
- aParticipants will complete disease-specific questionnaires based on primary diagnosis. CAN care assessment need, CCQ Clinical COPD Questionnaire, COPD chronic obstructive pulmonary disease, EMR electronic medical record, FACT-G Function Assessment of Cancer Therapy—General, GAD-7 Generalized Anxiety Disorder-7, GSDS General Symptom Distress Scale, ILD interstitial lung disease, ISI Insomnia Severity Index, K-BILD King’s Brief Interstitial Lung Disease, KCCQ-12 Kansas City Cardiomyopathy Questionnaire, PEG a three-item scale assessing pain intensity and interference, PHQ-8 Patient Health Questionnaire-8, PROMIS Fatigue patient-reported outcomes measurement information system fatigue scale, QUAL-E Quality of Life at the End of Life